看

Research projects

看
看

Single-molecule manipulation with optical tweezers
Actomyosin motility
Molecular biophysics of titin
Nanomechanics of fibrous biomolecular systems
Nanobiotechnology applications with AFM
Instrumentation development
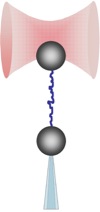

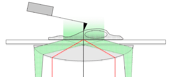
We explore, with a dual-beam counter-propagating optical tweezers instrument, the elasticity, force-driven structural transitions (unfolding), and dynamic interactions in various biomolecular systems (titin, actin, DNA, chromatin). The image shows the schematics of the experimental layout in which a filamentous molecule, held by its ends between a trapped bead and a bead captured with a moveable micropipette, becomes stretched. The movie is the recording of a DNA-stretching experiment.
看
看
看
看
看
看
The function of motor proteins, such as myosin, is investigated using the in vitro motility assay. The movie shows the sliding motion of individual, fluorescently labeled actin filaments over a field of myosin molecules. We study the motile features of myosin extracted from human biopsy samples (cardiac and orthopaedic surgery specimens), cloned myosin molecules and fragments.
Titin is a giant filamentous muscle protein, the largest single polypeptide known to date. It defines the elasticity of our muscles. It also plays a role in regulating contraction and sensing muscle contractile status. We study the elasticity, stability and interaction of titin and its recombinant domains by using optical tweezers, AFM and microscopic, spectroscopic and biochemical assays. On the right the AFM image of a titin oligomer is shown. Click on it to listen to the music of titin as its globular domains pop open during force-driven unfolding. Below is a model of the force-driven unfolding and refolding processes in titin.
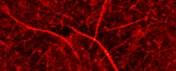
In order to expand the temporal and spatial scales of our analyses, we continually improve our methodology. Among others, we developed a spatially and temporally synchronized AFM and TIRF instrument capable of recording the mechaics/topograpy and fluorescence of the sample at the same time. On the right the schematics of this instrument is sown, along with an image of a cultured HeLa cell labeled with rhodamine-phalloidin. The color scale corresponds to fluorescence intensity (yellow is more intense).
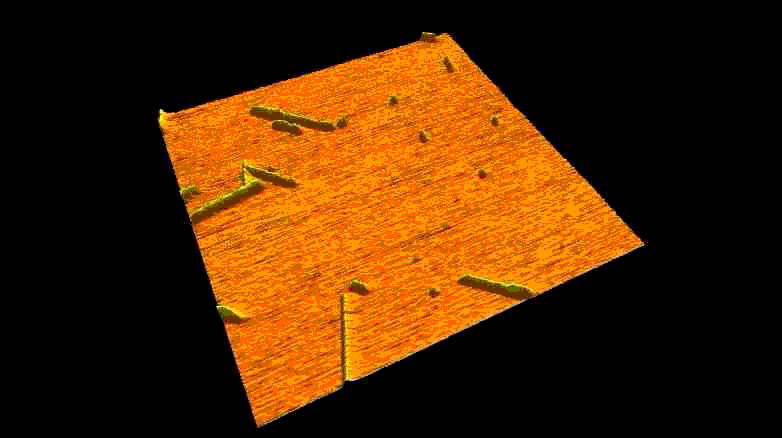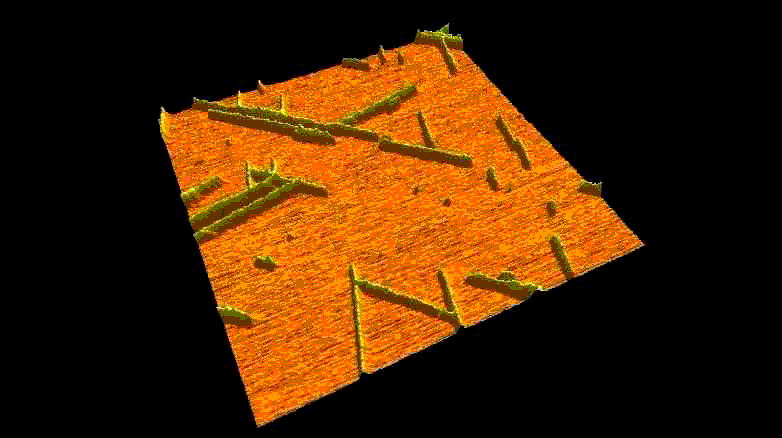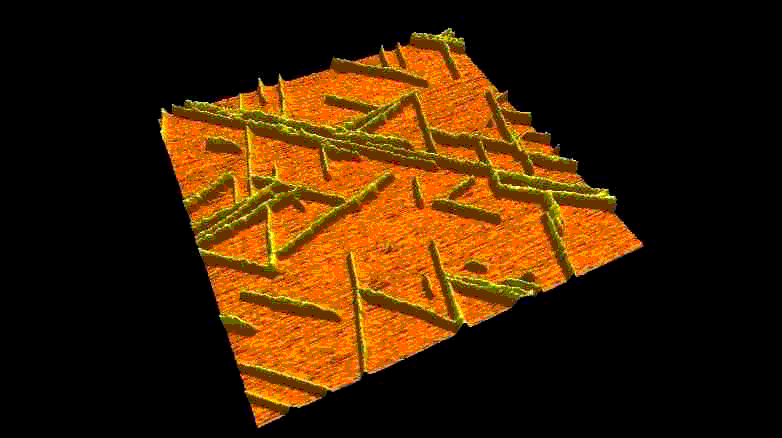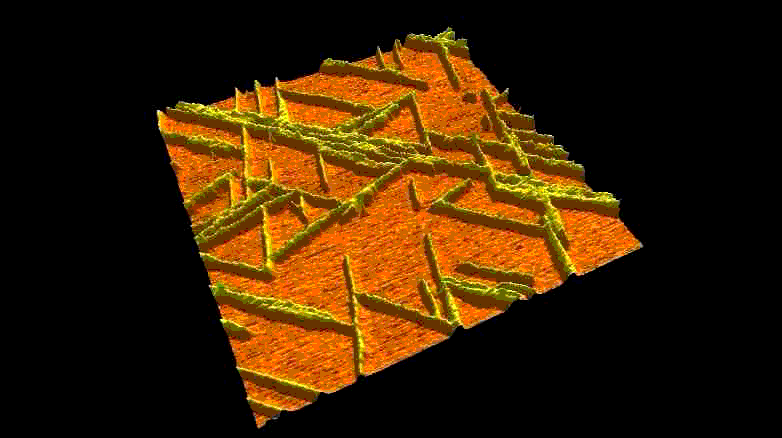
Fibrous biomolecular systems play a very important role in biology, yet their mechanics, interactions, mechanisms of formation are little understood and often dificult to investigate. We study various filamentous systems such as amyloid fibrils (above), fibrin (below), desmin intermediate filaments, collagen, bacterial flagella and myosin thick filaments. By mechanically manipulating such fibers we can unzip their protofilaments and explore the nature of interactions holding the supramolecular complexes together.
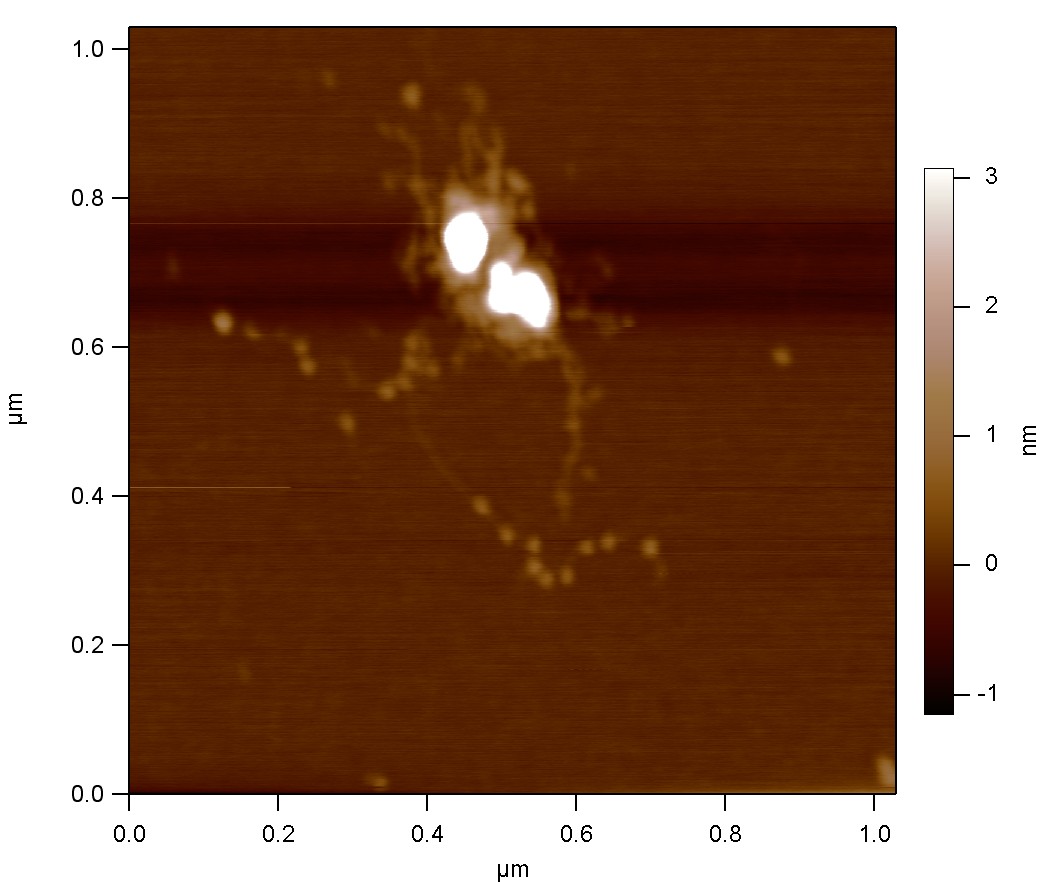
In our working definition, nanobiotechnology is the combination of biotechnology (recombinant) tools with single-molecule methods (manipulation and imaging). We develop tools that enable us to specifically grab and hold individual molecules for subsequent imaging and manipulation. Furthermore, we utilize our findings (e.g., amyloid growth mechanisms, right) towards potential applications in nanoelectronics and nanosensing.
看

