Molecular mechanism and interactions of SLUT1 enzyme - Dániel Tóth's PhD defense
2024. december 4
PhD-defense of Dr. Dániel Tóth will be held on the 9th December 2024. in the library of the Department of Physiology, EOK, Semmelweis University.
Comittee: Dr. Romána Zelkó (chair), Dr. András Czirók, Dr. Christian Jelsch. Opponents: Dr. Nathalie Lagarde, Dr. Zoltán Gáspári.
Supervisors of the candidate: Dr. Erika Balog, Dr. Maria Miteva (Univ. Paris Cite).
Thesis Title:
Molecular mechanisms of phase II drug metabolizing enzymes SULT1 and their interactions with small ligands modeled through computational approaches
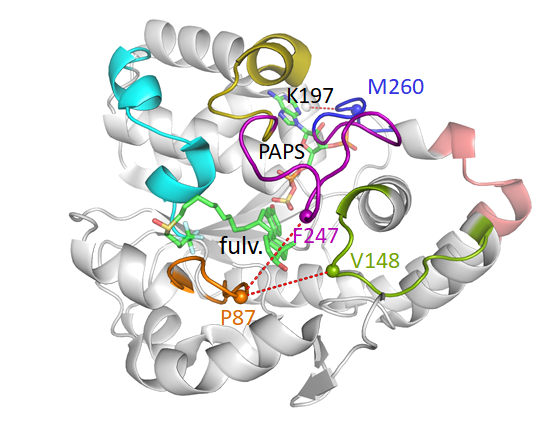
Abstract:
A major challenge associated with identifying promising drug candidates is to find a good balance between the required efficacy, selectivity and affinity against its intended therapeutic target while also showing an appropriate absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) profile. A high percentage of drug candidate failures are due to toxicity or undesirable drug-drug interactions (DDIs), many of these are due to the inhibition of Drug Metabolizing Enzymes (DMEs). Sulfotransferases (SULTs) are one such family with a wide variety of endogenous compounds and drugs. Although SULT1A1 and SULT1A3 share 93% identity, SULT1A1, exhibits a broad substrate range, while SULT1A3 displays a high affinity toward monoamine neurotransmitters like dopamine. The first aim of the thesis was to elucidate the selectivity of two major SULT1 isoforms, SULT1A1 and SULT1A3, by combining different in silico methods. To understand the factors determining the substrate specificity of the SULT1 isoenzymes, we studied the dynamic behavior and structural specificities of SULT1A1 and SULT1A3 by using molecular dynamics (MD) simulations and an enhanced MD approach, MD with excited Normal Modes (MDeNM). Additionally, we performed ensemble docking of common and specific substrates of the two isoforms. These results helped us to elucidate molecular mechanisms guiding the recognition of diverse substrates and inhibitors of SULT1. We identified key protein residues strongly involved in the recognition of different substrates for the two isoforms. Our analyses indicated that being more specific and more flexible, the structure of SULT1A3 has particularities in the binding site, which are crucial for its substrate selectivity. Our second main objective was to better understand how the dimerization is involved in their specificity. Performing comparative MD simulations for SULT1A1 as a monomer and as a dimer, with bound cofactor PAPS and the bulky substrate fulvestrant, revealed unexpected interactions within the dimer, and a clear influence of the dimerization on the conformational behavior of the enzyme, which is strongly related to substrate binding. Our results shed new light on the molecular mechanisms involved in the specificity of SULT1 family important for the metabolism of endogenous compounds, drugs, and DDIs.

