MemMoRFs, MemBlobs and conftors: Computational studies of membrane proteins - PhD-defense of Georgina Gáspárné Csizmadia
2025. április 23
PhD-defense of Georgina Gáspárné Csizmadia will be held on the 5th May 2025. in the Simonyi Room of Department of Internal Medicine and Oncology, Semmelweis University. The defense will be held in Hungarian.
Comittee: Dr. Péter Várnai (chair), Dr. Miklós Cserző, Dr. Imre Jákli. Opponents: Dr. Gábor Turu, Dr. Zoltán Gáspári.
Supervisors of the candidate: Dr. Tamás Hegedűs.
Thesis Title:
MemMoRFs, MemBlobs and conftors: Computational studies of membrane proteins
Abstract:
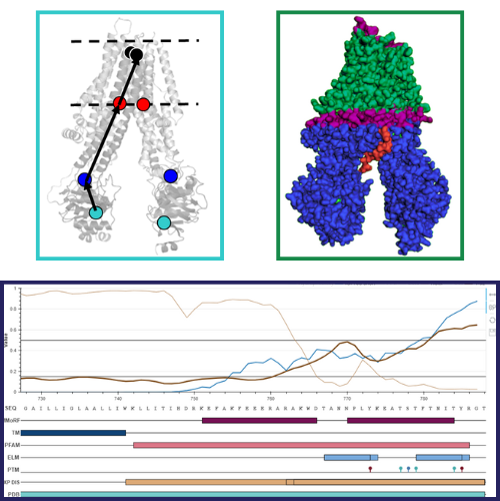 This thesis outlines three computational studies on membrane proteins, emphasizing the crucial need to comprehend their diverse conformations for deciphering functional mechanisms during the transport cycle. Despite the significance of studying transmembrane regions, sparse experimental data exists on membrane embedment. Intrinsically disordered regions in membrane proteins may impact function through membrane bilayer interaction, yet detailing these structural aspects poses a challenging gap in existing literature. My aim was to fill these gaps using computational methods.
This thesis outlines three computational studies on membrane proteins, emphasizing the crucial need to comprehend their diverse conformations for deciphering functional mechanisms during the transport cycle. Despite the significance of studying transmembrane regions, sparse experimental data exists on membrane embedment. Intrinsically disordered regions in membrane proteins may impact function through membrane bilayer interaction, yet detailing these structural aspects poses a challenging gap in existing literature. My aim was to fill these gaps using computational methods.
First, I developed computational methods to assess the new structures of Pgp-like membrane proteins, which are constantly increasing in number. I showed that structure-specific conformational vectors called conftors are suitable to describe the differences in conformations, to compare structures, to highlight inaccuracies, and to analyze molecular dynamic simulations.
Second, I aided the development of the MemBlob pipeline, which provides experiment-based transmembrane regions for transmembrane proteins solved by cryo-EM that is an entirely new addition to the field. The analysis of the resulting transmembrane regions showed that the thickness of the membrane blob could be affected by the membrane mimetics used during structure determination.
Finally, I collected, categorized and analyzed the data of membrane interacting intrinsically disordered regions (MemMoRFs) on which a database was built. This study showed that disordered regions tend to form helical structures during membrane interaction and that this interaction can be regulated by phosphorylation. As several pathogenically relevant proteins contain MemMoRFs, these regions could be potential therapeutic targets.

